Study Points
- Back to Course Home
- Participation Instructions
- Review the course material online or in print.
- Complete the course evaluation.
- Review your Transcript to view and print your Certificate of Completion. Your date of completion will be the date (Pacific Time) the course was electronically submitted for credit, with no exceptions. Partial credit is not available.
Study Points
Click on any objective to view test questions.
The ACAM2000 smallpox vaccine contains the live virus
Click to ReviewThe smallpox vaccine does not contain smallpox (variola) virus. It contains vaccinia and protects against orthopox diseases, such as smallpox (variola), cowpox, monkeypox, mousepox, and rabbitpox. Dryvax and Wetvax, which were formerly the only smallpox vaccines available in the United States, have been superseded by ACAM2000, a "second-generation" vaccine derived from a clone of Dryvax, purified, and produced using modern cell culture technology, and JYNNEOS, a "third-generation," highly attenuated vaccine. The manufacturer of ACAM2000 has stated that it will provide the vaccine only for Strategic National Stockpile (SNS) use [4,5]. The SNS is a national repository of life-saving pharmaceuticals and medical material maintained by the CDC; it includes a post-event smallpox vaccine inventory. More modest supplies of the vaccine, reserved for pre-event vaccination efforts, are maintained by state and local health agencies, as recommended by the CDC. The SNS program works with government and nongovernment partners to upgrade the nation's public health capacity to respond to a national emergency. Critical to the success of this initiative is ensuring that capacity is developed at the federal, state, and local levels to receive, stage, and dispense SNS assets [6].
For medical personnel, vaccination against smallpox is currently
Click to ReviewAll medical personnel should think through their volunteer participation in a pre-event vaccination program. Because the risk of smallpox occurrence is an unknown factor, as is the possibility of its being used as a weapon of bioterror, the risk/benefit equation that one would normally weigh when considering whether to receive an immunization does not apply. The primary benefit from choosing to receive the vaccine would be the protection of the nation. Personal benefit would likely be realized only in the event of exposure to smallpox; therefore, each healthcare professional should weigh the likelihood of personal exposure. Personnel working in critical care, emergency response, or public health are more likely to be exposed than those on a surgical ward. Obviously, members of the public health response teams and care provider teams should be vaccinated if they have no contraindications. Vaccinators are assumed to have a certain level of risk; protection cannot be considered absolute [7].
Smallpox is transmitted
Click to ReviewAs previously mentioned, smallpox is transmitted mainly from person to person by the respiratory route through droplets. Close personal contact (normally within six feet of a patient) over a prolonged period, such as in a household, is required for transmission to occur. Smallpox is not as infectious as measles, chickenpox, or influenza. As a result, transmission in the workplace and school is less than in the household [23]. Data collected in the 1960s showed that an estimated 80% of the cases were contracted from an individual in the household [23]. In this course, "household contact" will be used in a broad sense and includes intimate and sexual contact. There is some indication in the medical literature that inanimate objects also may transmit the disease, but this is less common [9,24,25]. Unfortunately, the use of variola as a biologic weapon is a threat because of the aerosol infectivity of the virus and the relative ease with which it can be mass produced [26].
Smallpox lesions differ from chickenpox lesions in that
Click to ReviewThe smallpox rash erupts at the end of the prodrome. A few lesions usually appear first on the face, especially on the forehead. These are called the "herald spots." Occasionally, the rash is first seen on the forearms. Lesions tend to appear on the proximal portions of the extremities, on the trunk, and then on the distal portions of the extremities. However, the rash usually progresses so quickly that within 24 hours it is apparent on all parts of the body, and the patient may not notice how the rash progressed. Normally, more lesions appear over the next one or two days, possibly followed by a few fresh lesions later. Generally, the rash is distributed in a centrifugal pattern. The rash is most dense on the face and is denser on the extremities than on the trunk. It is more prominent on the distal regions than on the proximal, and on the extensor rather than on the flexor surfaces. There also may be lesions on the palms and soles [31].
The classic smallpox lesions are round, well-circumscribed vesicles progressing to pustules that are deep-seated and firm. They feel "shotty" (round and hard) when rolled between the thumb and forefinger. As they continue to develop, the lesions become umbilicated, having a central "naval-like" depression. The more confluent the lesions, the more grave the prognosis. One of the distinguishing features of smallpox rash is that the lesions on any specific area of the body are all in the same state of development, meaning that they are all vesicles, pustules, or umbilicated lesions. In contrast, the rash of chickenpox starts as a vesicle on top of erythema—the "dewdrop on rose petal." Chickenpox come in "crops," so in any one area of the body there will be vesicles, pustules, and crusts (scabs). The palms and soles are rarely involved, and patients are rarely toxic or moribund [30].
Following the ring vaccination protocol means vaccinating
Click to ReviewThe purpose of ring vaccination is to form a buffer of immune individuals around the organism to prevent the spread of the disease. It is incorrect to illustrate this concept by drawing a "ring" on a map, within which all individuals would be vaccinated. Ring vaccination is not a geographic designation. It is a social designation in which a "ring" is drawn around each smallpox victim, and it includes close household contacts who may have been exposed to the victim. (The victim must be isolated to limit the number of contacts.) There is a three- to four-day window in which a person exposed to smallpox may be vaccinated to prevent the disease, or at least make the symptoms milder. To help eradicate the disease, a second "ring" also is vaccinated. The second ring consists of those who have had contact with the individual(s) exposed to the smallpox victim and the members of their households [42]. These individuals also would need to be vaccinated because it is not possible to know exactly when a contact was exposed and whether the three- or four-day period had already lapsed. Contacts would be vaccinated up to seven days after exposure. They would still likely develop smallpox, but it would be less severe [17]. For anyone exposed to a smallpox patient, the pre-event contraindications will be modified, as the disease has a fatality rate of 30% [9]. This would mean 300,000 fatalities in one million cases of smallpox, compared to one to two fatalities in one million vaccinations.
Which of the following is an FDA-approved smallpox vaccine?
Click to ReviewIn 2007, prior to the removal of Dryvax from the SNS and other programs, the FDA licensed ACAM2000 (Sanofi Pasteur Biologics, formerly Acambis), a second-generation smallpox vaccine, for use in the United States. ACAM2000 is a live vaccinia virus derived from plaque purification cloning from Dryvax. Like Dryvax, ACAM2000 is contraindicated for immunocompromised individuals and has been associated with serious side effects, such as myocarditis, encephalitis, and ocular complications. Based on clinical studies, myocarditis and/or pericarditis have occurred in 1 in 175 adults who receive the vaccine for the first time [4]. Serious health problems, including those that are life-threatening, also may occur in unvaccinated individuals who are accidentally infected by someone who has recently been vaccinated [4]. The percentage of unvaccinated persons who develop a successful immunization reaction from ACAM2000 has been shown to be similar to that of Dryvax. ACAM2000 also has been found to be acceptable as a booster in those previously vaccinated for smallpox. It is important to note, however, that a 2008 study examining the continued immunity conferred by smallpox vaccination administered in childhood found that 97% of participants showed no decrease in vaccinia antibody titers (after a range of 13 to 88 years) [46]. As a result, there may be no need for booster vaccinations, and the available supplies of vaccinia may be better used in those who have not previously been vaccinated.
Vaccinia produces
Click to ReviewLike many vaccines, vaccinia produces both humoral and cell-mediated immunity [14]. The humoral response results in the production of short-lived IgM antibody, persistent IgG antibody, and other types of antibodies. A convenient memory clue when reading laboratory results for infectious diseases is that IgM could mean "I get medicine," which indicates the short-lived antibody and is a marker for acute infection. Of course, there may be no medicine or treatment available, but this person is probably contagious if they are still exhibiting symptoms. IgG could be thought of as "I get germs" and indicates a persistent antibody that confers some degree of immunity. The person with these laboratory results has had the infection in the past and would not be a candidate for any available treatment or medicine, nor would that person be contagious. This is especially significant in reading laboratory results for a patient with a suspected disease, such as hepatitis A.
In the pre-event vaccination program, which of the following candidates should NOT be vaccinated with ACAM2000?
Click to ReviewIn a pre-event program, smallpox vaccination has not been recommended for anyone younger than 18 years of age and is contraindicated for persons with any of the following [7,65,66]:
History or presence of eczema or atopic dermatitis
Other acute, chronic, or exfoliative skin conditions
Conditions associated with immunosuppression
Therapy with alkylating agents, antimetabolites, radiation, tumor necrosis factor inhibitors, and/or high-dose corticosteroids
Hematopoietic stem cell transplant recipients
Autoimmune disease (e.g., systemic lupus erythematosus)
Age younger than 1 year
Serious allergy to any component of the vaccine
Pregnant or breastfeeding
Heart disease or three or more risk factors for cardiac disease
A candidate should not be vaccinated if he/she has a household contact who
Click to ReviewBecause vaccinia is a live vaccine and will be shed from the vaccination site, household contacts should be considered at risk if they have any contraindications to the vaccine. When screening for a pre-event program, household contacts include persons with prolonged intimate contact (e.g., sexual contact) with the potential vaccinee as well as anyone who might have direct contact with the vaccination site. The contraindications for household contacts include [7]:
Skin conditions (as listed previously)
Any immunosuppression due to HIV/AIDS, generalized malignancy, leukemia, lymphoma, solid organ or stem cell transplantation, humoral or cellular immunity disorders
Autoimmune diseases
Pregnancy
Heart disease, such as coronary artery disease and congestive heart failure, or three or more risk factors for cardiac disease, such as diabetes, high cholesterol, and high blood pressure
A primary vaccination with ACAM2000 means that the vaccinee
Click to ReviewThe vaccinator's free hand should grasp the vaccinee's upper arm in a way to make the skin taut. Preferably, the arm should be held from underneath as the skin may be held taut effectively, and the vaccinator's hand may be protected from an accidental needle stick. The wrist of the hand holding the needle of the vaccinator should rest against the vaccinee's arm [66]. The vaccinator should rapidly make 15 jabs of the needle perpendicular to the skin through the vaccine droplet (approximately a 2.5-mcL dose) to puncture the skin, within a diameter of about 5 mm. The jabs should be vigorous enough to bring a drop of blood, after 15 to 20 seconds, at the vaccination site [66]. ACAM2000 uses 15 insertions for both primary (has not been previously vaccinated) and revaccination and has no provision for additional insertions if no trace of blood is visible after vaccination [8]. The needle should not be redipped into the vaccine after it has touched the vaccinee's skin. After use, the needle should be discarded in a suitable biohazard receptacle [66].
The double-layered dressing
Click to ReviewWhen working in a healthcare setting, vaccinees should cover the vaccine site with gauze and a semipermeable dressing placed over the gauze. The semipermeable dressing used must be one in which there are no added compounds, such as a microbicide. When placing the semipermeable dressing, make sure that all the edges adhere well to complete the seal. If not, reinforce the edges. Products that combine an absorbent base with an overlying semipermeable layer also may be used to cover the vaccination site. Healthcare workers do not need to be placed on leave after receiving a smallpox vaccination [66].
Effective handwashing
Click to ReviewTo remove the oils and organisms, soap must be used and the hands should be well lathered, vigorously rubbed, and then rinsed well under running water. A minimum of 20 seconds of lathering, rubbing, and rinsing are needed to assure that the oils and organisms are removed (imagine singing "Happy Birthday" twice through). Warm water, if available, is suggested for comfort, but the water used for hand washing would never be hot enough to kill the organisms. Rinse hands well under running water and then dry, using either a paper towel or an air dryer. Ideally, use the paper towel to turn off the faucet and then discard the towel appropriately; using an elbow to turn off the faucet is an alternative [75,76].
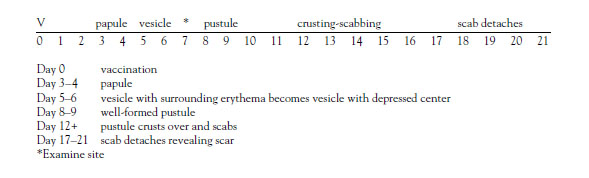
The optimal day for reading the site to determine if the vaccination is effective is
An equivocal reaction indicates that the
Click to ReviewOn the seventh day following vaccination, if the site does not have the vesicle, it is termed "equivocal" (a"non-take"). Equivocal reactions consolidate a variety of previously used terms, including accelerated, modified, vaccinoid, immediate, early, or immune reactions. Equivocal reactions have been defined as all responses other than "major reactions." There also may be no reaction at all. About 5% of vaccinations are equivocal [74]. There may also be an allergic reaction, which does not indicate immunity. An equivocal reaction means that there is no immunity and the vaccination must be repeated, preferably immediately after the evaluation. Usually, the second vaccination results in a take, but occasionally there is an equivocal reaction or no reaction even with the second vaccination. Equivocal readings may be caused by a number of factors, including improper vaccination technique, use of vaccine that has lost its potency, or residual immunity among previously vaccinated individuals. Persons with an equivocal reaction cannot be presumed to be immune to smallpox; revaccination is recommended [79].
A common systemic effect of vaccination with vaccinia is
Click to ReviewSystemic symptoms are expected and usually occur 8 to 10 days after vaccination when the vaccine site reaction reaches the peak of the inflammatory response. Occurrence of the following symptoms has been shown to vary considerably between primary vaccinees (higher rates) and revaccinees (lower rates) [78,81]:
Intense erythema ringing the vaccination site
Malaise
Myalgia, headache, chills, nausea, fatigue (0.3% to 37%)
Fever >37.7°C (2% to 16%)
All of the following are common sites of autoinoculation, EXCEPT:
Click to ReviewAutoinoculation is the transfer of or appearance of vaccinia lesions elsewhere on the body. Because vaccinia is shed from the site after the vesicle appears and until the scab falls off, a vaccinee whose hand hygiene is inadequate may inadvertently inoculate other sites on his/her person. The most common sites are the face, eyelid, nose, mouth, genitalia, and anal region [80,81]. Infants and children are most susceptible because they tend to scratch the itching vaccination site [81]. Skin with disrupted integrity and the eyes are especially vulnerable as the infection may be extensive in skin lesions and a threat to eyesight. Data from the 1960s have revealed that accidental implantation of vaccinia is the most frequent complication of vaccination (i.e., 25.4 per million primary vaccinees, which accounted for approximately one-half of all the complications of primary vaccination and revaccination) [82]. This complication highlights the need for a proper dressing on the site and a sleeve that prohibits scratching, as well as attention by the vaccinee to good hand hygiene [80].
The recommended treatment for vaccinia keratitis is
Click to ReviewVaccinia keratitis is a rare complication that results from the introduction of vaccinia from the vaccination site to the cornea. It is characterized by corneal lesions, abrasions, ulceration, and subsequent cloudiness [80,81]. The lesions are often indurated, edematous, and infiltrated. If the condition progresses untreated, it may lead to scarring and permanent impairment of vision. Individuals with pre-existing eye conditions, especially those that are inflammatory in nature, are more susceptible to implantation. Those who care for children who have received vaccinia are the most likely to develop vaccinia keratitis [80]. An ophthalmology consultation should be obtained for evaluation and management of keratitis and other eye complications resulting from inadvertent ocular implantation of vaccinia virus. The best method for diagnosis and monitoring of changes and response to treatment is slit-lamp evaluation. Treatment should include topical antiviral medications as advised by an experienced ophthalmologist. VIG is contraindicated and, if used, may increase the risk of scarring [83].
Generalized vaccinia
Click to ReviewGeneralized vaccinia (GV) is rare and probably caused by viremia. It consists of vesicles or pustules appearing on normal skin far from the vaccination site. The 1960s data has estimated that GV will occur in 242 per million primary vaccines [82]. Rates in the United States today may be higher because there may be more persons at risk from eczema or atopic dermatitis and immune suppression from cancer, cancer therapy, organ transplantation, and other illnesses (e.g., HIV/AIDS) [80]. The disease is usually mild, self-limited, and has little residual damage. Mild cases require no treatment, but more extensive lesions, especially in a vaccinee with an underlying immunosuppressed illness, may be toxic and treatment with VIG will be necessary [80,81,82].
Historically, life-threatening events following vaccination occurred
Click to ReviewIn this course, adverse events are defined as life-threatening and include eczema vaccinatum, vaccinia keratitis, progressive vaccinia, post-vaccinial encephalitis, and fetal vaccinia. In the past, between 14 and 52 vaccinees per million vaccinated have had life-threatening reactions to vaccinia, and 1 to 2 died [82].
Vaccinia immune globulin is
Click to ReviewVIG has been used in the past and was felt (but not shown through controlled studies) to be effective. VIG was originally produced in the 1960s from plasma obtained from recently vaccinated donors. Like other globulins, VIG provides passive immunity. Because of the high proportion of aggregated protein, it was originally administered solely by the intramuscular route; it could not be given intravenously [82,83].
In 2005, the FDA approved CNJ-016, vaccinia immune globulin intravenous (human) (VIGIV), for use in the treatment of adverse events resulting from the administration of vaccinia [87]. VIGIV has a low level of aggregated protein, so it may be used both intravenously and intramuscularly [80,83]. Absolute contraindications for the use of VIGIV include hypersensitivity to immune globulin or any component of the formulation, isolated vaccinia keratitis, and selective IgA deficiency [70,87]. The dosing recommendation for CNJ-016 (the Cangene product) is 6,000 units/kg; however, 9,000 units/kg may be considered if the patient does not respond to the initial dose. The dosing recommendation for the DynPort product is generally 100 mg/kg; however, higher doses (200–500 mg/kg) may be administered if the patient does not respond to the initial recommended dose [70].
VIG has not been recommended for the following reactions [70,83]:
Autoinoculation with few lesions
Bacterial infections
Erythema multiforme
Generalized vaccinia that is mild and limited
Vaccinia keratitis
Post-vaccination encephalitis
VIG has been recommended for the following reactions [70,82,83]:
Autoinoculation with extensive lesions
Eczema vaccinatum
Generalized vaccinia that is severe or recurrent
Progressive vaccinia/vaccinia necrosum
- Back to Course Home
- Participation Instructions
- Review the course material online or in print.
- Complete the course evaluation.
- Review your Transcript to view and print your Certificate of Completion. Your date of completion will be the date (Pacific Time) the course was electronically submitted for credit, with no exceptions. Partial credit is not available.